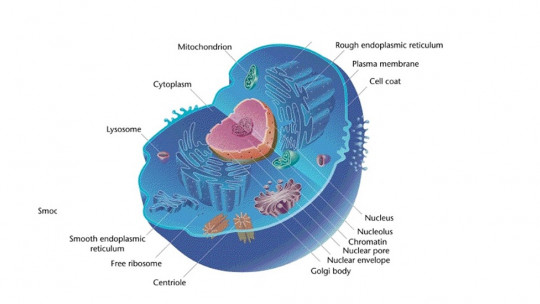
Proteins are macromolecules made up of amino acids. About 500 different amino acids have been described in nature, but curiously, only 20 are the essential ones present in the human body. DNA contains all the information necessary for a protein to be synthesized, since through transcription and translation mechanisms, a triplet of DNA nucleotides is converted into a specific amino acid.
Ribosomes are the organelles responsible for assembling these amino acids, giving rise to chains with variable orders and length, or in other words, what we know as proteins. These biomolecules are essential for conceiving life, as they represent approximately 80% of the dry protoplasm in every cell and represent 50% of the weight in all living tissues.
With these data in hand, it is more than clear to us the importance of proteins in the generation of life. Today we come to bring you a very interesting mechanism related to this topic, because we tell you everything about it. Bradford’s method designed to quantify the protein concentration of a solution.
What is the Bradford method?
The Bradford method (known as Bradfords protein essay in English) was described, as its name indicates, by the American scientist Marion Mckinley Bradford, in 1976. First of all, it is necessary to highlight that It is a spectrometric method, a term that encompasses a set of laboratory procedures based on the interaction of electromagnetic radiation with an analyte (the component of interest that you want to separate from the matrix).
In addition to this, it should be noted that it is a colorimetric method, that is, it obtains results based on the colors and their concentration in a specific solution The key to this terminological conglomerate is found in the “Coomassie blue” dye, since in the Bradford method the changes in its absorbance are quantified according to certain parameters. This dye appears blue in its anionic form, green in its neutral form, and red in its cationic form.
Under acidic conditions in the solution, Coomassie blue turns from red to blue and, in the process, binds to the proteins to be quantified. If there are no proteins in the aqueous medium, the mixture remains brown, so it is very easy to detect the presence of these macromolecules in the first instance with this methodology.
The chemical bases of the Bradford method
We enter into a little more complex terrain, since it is time to describe what happens between these molecules beyond direct color changes. When joining with the protein, Coomassie blue in its cationic and double protonated form (red) forms a very strong non-covalent bond with said macromolecule through van der Waals forces and electrostatic interactions.
During the formation of this chemical complex, the dye donates its free electron to the ionizable portions of the protein (we remember that cation = positive charge, loses electrons), which causes the disruption of the normal protein state. This exposes certain substances that can generate the previously described unions, which we will not dwell on due to their chemical complexity. In summary, you only need to know the following:
Red dye (cationic/non-protein bound) ≠ Blue dye (anionic/protein bound)
Based on this premise, it is worth noting that The red dye has an absorption spectrum of 465 nm, a value that represents the incident electromagnetic radiation that a material absorbs within a range of frequencies In the anionic blue form (interacting with proteins), a change in absorption occurs at 595 nm. Therefore, in a solution subjected to the Bradford method, readings are made in spectrophotometers at a range of 595 nm.
The increase in absorbance in this spectrum is directly proportional to the number of bonds between the dye and the proteins, so not only is it detected that there are proteins with the color change, but it can also be estimated how much protein there is per milliliter of liquid medium. Incredible true?
Bradford method procedure
In order to carry out this methodology, a spectrophotometer is necessary, which is not exactly cheap (approximately 2,000 euros), so it is not something that can be executed from home This machine is capable of projecting a monochromatic beam of light through a sample, in order to measure the amount of light that is absorbed by the compounds of interest. Thus, the researcher receives information about the nature of the molecules in the solution in question and, incidentally, is also able to calculate the concentration of said molecule.
Furthermore, it is necessary to emphasize that the reagent is not only “raw” Coomassie blue. 100 milligrams of the dye should be dissolved in 50 milliliters of a 95% ethanol solution and add 100 milliliters of 85% phosphoric acid. In addition, it is necessary to dilute it to one liter once the dye has dissolved and filter the mixture, to give rise to the final reagent used in the method. The color of this solution without proteins present, as we have said, should be brownish
Once the researcher has the reagent and the spectrophotometer, they must follow the following steps:
The results will appear on the spectrophotometer screen, and must be recorded by the professional who is carrying out the research. Once you have them, It is necessary to create a graph (calibration curve) that faces two values on their axes: absorbance vs micrograms of protein From the curve generated with the values, these can be extrapolated to obtain the exact concentration of protein in the solution.
Advantages
The Bradford method is very easy to execute for anyone related to the laboratory field, since every biologist and chemist has faced a spectrophotometer at least once during their years of study. Whether it is measuring the amount of chlorophyll in a solution from crushing a leaf (typical) to much more complex things, spectrophotometers are widely used in learning environments.
In addition to its ease, It should be noted that many proteins in their natural state have an extremely low absorption range, at 280 nm Not even all proteins reach this value, since to do so they must present specific amino acids (tyrosine, phenylalanine and tryptophan), which are not always present. Since this absorbance figure is in the UV range, a special machine is necessary that almost no one has to be able to treat them.
Really, What is done in the Bradford method is to “increase” the absorbance value of the proteins by binding to a dye In addition to being much easier to read in this state, proteins move away from the absorbance spectra of other biological molecules, which could contaminate the sample.
Summary
In this small chemistry class, we have immersed ourselves in one of the simplest and easiest protein quantification methods to perform, as long as you have the relevant material. In any case, we must highlight that, like everything in this life, it is not perfect or infallible: it is usually necessary to make multiple dilutions of the sample for analysis (minimum and maximum values from 0 µg/mL to 2000 µg/mL), which which can lead to mistakes during the process.
Furthermore, the presence of detergents and other compounds in the solution can prevent the correct development of the method. Luckily, there are other reagents that can be added to the mixture to solve these problems in many cases.