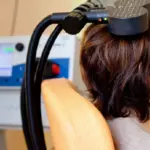
In clinical trials with a randomized control group, it is appropriate to measure the extent to which the belief in receiving the experimental treatment influences the degree of improvement reported by the volunteers.
The placebo effect is widely known in research, which can be defined as the improvement perceived by participants, who believe they have received the effective treatment, even though this is not the case.
However, the placebo effect is not the only one that can occur in this type of trials. The lessebo effect, along with the nocebo, are also a product of suggestion Next we will see what the lessebo effect is, in addition to relating it to the other two.
The lessebo effect and relationship with research
In science, when creating a new clinical intervention, whether it is a drug, a new type of therapy or any new treatment, it is necessary, first of all, to check if it really works. For this purpose, it is common to carry out clinical trials, in which volunteer participants who have the medical or psychiatric condition that the new intervention is believed to improve will participate.
However, to correctly detect the therapeutic capacity of the new intervention, it is normal that in these trials there are, at least, two groups: one experimental and one control The experimental group will be made up of participants who are going to receive the intervention, with the intention of seeing what effects it has on their health, whether there is improvement or worsening of symptoms. On the other hand, the participants in the control group will not be given any therapeutic treatment. Both the participants in the control group and those in the experimental group do not know which group they were assigned to.
The objective of forming these two groups is to know to what extent the improvement (and also worsening) of the participants is attributable to the application of the intervention
The idea is that if there is improvement in the experimental group and not in the control group, the improvement is attributable to the treatment. If there is some type of improvement in both groups, this will not be related to the intervention, but rather attributable to the course of the medical or psychiatric condition that is intended to be treated. Indeed, there are medical illnesses and mental disorders that can improve simply with the passage of time.
Let’s start at the beginning: the placebo effect
Up to this point everything makes sense, but surely one question comes to mind: If the experimental group receives the treatment to be tested, what does the control group receive? The volunteers in the control group have to receive something, otherwise they will know that they are in such a group and it is something we do not want. What we want in research is to verify the pure and simple effectiveness of the treatment, and for this we need that those who are receiving it do not know that they are receiving it but show improvement if it is effective.
For this reason all participants in the experiment receive something. If the experimental treatment is applied to the experimental group, a placebo is applied to the control group. A placebo substance or treatment is any intervention that those who apply it know or assume will be It does not have any type of effect, neither therapeutic nor harmful For example, in pharmaceutical research, if the experimental group is given the drug that is believed to work, the control group will be given something that looks like a drug, in the form of a pill or syrup, but without any active component.
And this is where we have to talk about the placebo effect. This effect is essential to be taken into account in research, given that it may well call into question the effectiveness of the new intervention. The placebo effect occurs when the control group, despite not receiving the experimental treatment, reports improvement The participants who form the control group have the expectation of having received the experimental treatment, and believe that it is being applied to them, perceiving an improvement that is nothing more than suggestion.
It is important to understand that before participating in an experiment, participants are given informed consent. It explains that the experimental treatment being tested can have both benefits and unwanted effects on health, and that the goal of the experiment is to discover what they are. Additionally, they are told that they may receive this treatment or they may be given a placebo. Despite knowing this information, it is not strange that participants want to be part of the experimental group, and believe that they have been assigned to that group, feeling a supposed improvement.
Use of placebo is the norm in randomized controlled trials The logic behind the application of placebos derives from the need to distinguish between the real benefit observed by the participant and the benefit that is a product of his desire to improve. The mind is very powerful and is capable of deceiving us, covering up symptoms and making us believe that we have improved.
Although the placebo effect has been known for quite some time and medical, pharmaceutical, psychological and psychiatric research has questioned it, the existence of two other effects given in an experimental context has been proposed: the nocebo effect and the lessebo. Both effects are very important, like the placebo effect itself, and can in fact bias the interpretation of the results of the experiment.
The nocebo effect
Before talking in more depth about the lessebo effect, it is worth briefly understanding what the nocebo effect is. “Nocebo” comes from Latin, meaning “I must do harm,” in contrast to the term “placebo,” which is “I must pleasure.” Knowledge of the nocebo effect is considered quite revealing about how everything related to the placebo (ineffective intervention) and its homonymous effect should be applied and interpreted, given that Even what should have no effect can cause harm
As we have already mentioned, the placebo effect is, in essence, the improvement perceived by the participants in the control group despite the fact that they have not been administered anything that is known to have any effect. The nocebo effect would be the opposite: it is the worsening of the symptoms or signs of a health condition due to the expectation, conscious or not, of undesirable effects of an intervention.
In experimentation there is always informed consent and, as we have previously commented, in it It is explained that the intervention can have positive effects and negative effects If the placebo effect is believing that you receive the intervention and have positive effects, in the case of the nocebo it is also believing that you are receiving that intervention, but that its adverse effects are manifesting. The participant has pessimistic expectations that make him believe that the treatment is harmful to him.
What characterizes the lessebo effect?
For a long time, research was only concerned with monitoring the suggestion and expectations of the control group, both positive and negative. Under the logic that something necessarily has to happen in the experimental group, both a therapeutic effect and adverse effects, the effects of suggestion in that same group were not monitored. Fortunately, although relatively recently, more attention has begun to be paid to how pessimistic expectations in the experimental group can nullify the actual therapeutic effects of the intervention.
If the placebo is the perceived improvement in the control group and the nocebo is the worsening, the lessebo effect is the perception of less improvement, cancellation of the effects or worsening in the experimental group That is, the participants in the experimental group, who are receiving the treatment, believe that they have either been given a placebo or are suffering the adverse effects of the treatment, believing that their condition is being worsened.
This It can be due to multiple causes It may be that, as would happen with the nocebo effect, participants have a pessimistic view of the effects of the experimental treatment, thinking that they are more likely to suffer its unwanted effects rather than its therapeutic ones. Another thing that has been seen is that there are many participants who, despite reading the informed consent, do not understand it, and think that “placebo” is synonymous with “harmful.” They think that the experimental treatment is beneficial and that the control is necessarily bad.
Scientific implications
It is clear that Both the placebo and nocebo effects affect research if they are not taken into account, but the lessebo effects are even worse As we have mentioned, it may be that the participant who is receiving an effective treatment thinks that it is not effective or that it is a placebo, and self-suggests himself to think that he is not improving or even getting worse.
Discarding something that, objectively speaking, is working but that volunteers report as harmful due to their pessimistic expectations not only implies discarding a treatment that works, but also implies loss of financial resources and time. Whether it is a drug, a new psychological therapy or any other type of treatment, its design and application involves the mobilization of many efforts, and for it to be discarded due to biases of the experimental participants is a real mistake.
It is for this reason that based on new research focused on studying the lessebo effect You should consider how trustworthy the participant is , in the sense of what kind of expectations you have about the experiment and whether you present an unrealistic style of thinking. Whether you tend toward pessimism or optimism, it is necessary to know this pattern of thinking, and find out to what extent that participant is not going to bias the results of the experiment.