According to the Global Burden of Disease Study, 95% of the world’s population has some health problem, at least in the sample group analyzed between 1990 and 2013. It is not surprising, since 15% of the biomass The world’s carbon in the form of carbon is made up of bacteria (70 gigatons), some of them beneficial to humans, others commensal and others directly pathogenic.
Beyond bacteria, there are thousands of non-living infectious agents in the form of viruses, which mutate at a frenetic rate and evolve to circumvent the long-term immunity of organisms. Human competition with pathogens is a true arms race: when a specific response is developed against a pathogen, it is expected that it will end up mutating so that it is no longer recognized by lymphocytes and other specific bodies.
For this reason, flu vaccination campaigns are annual, while other vaccines provide lifelong immunity against a given pathogen. Depending on the mutation rate and adaptability of the microorganism, the chances of infection may increase or decrease over time. Based on these very interesting premises, we tell you everything you need to know about immunoglobulins
What are immunoglobulins?
According to the National Cancer Institute (NIH), an immunoglobulin or antibody is a protein made by plasma cells (types of white blood cells) in response to the presence of an antigen, a substance that causes the human immune system to activate, recognizing itself as a threat. The key to understanding immunity is based on the antibody (Ig) – antigen (Ag) dyad or, in other words, Ig-Ag.
Each immunoglobulin binds to a single antigen, allowing immune cells specialized in destruction (such as macrophages) to more effectively recognize the pathogen and phagocytose it, but some of them can also destroy the antigen directly Each antibody has a specialized paratope or antigen binding site, which is specific to the epitope of the antigen itself. In other words, each Ig-Ag complex represents a non-transferable key and lock.
The clearest use of immunoglobulins in general society is, without a doubt, the development of vaccines. When a weakened virus or bacteria is introduced into the body (or a section of it that promotes an immune response), it stimulates the proliferation of lymphocytes and the release of immunoglobulins specific for said antigen. So, the body “learns” which microorganism is dangerous, always from the safety of prior pathogenic inactivation
Thanks to this safe immunization mechanism, it is estimated that more than 37 million lives have been saved around the globe in the last 20 years, especially in children. A clear example of this is smallpox: in the 18th century, 400,000 people died annually from this disease, which translated into a fatality rate for the agent of almost 30%. Thanks to vaccination, the last case of smallpox was diagnosed in 1977, and the WHO declared the world free of the pathogen in the 1980s. Without a doubt, knowledge of immunoglobulins has allowed us as a species to free ourselves from epidemiological havoc.
Structure of these proteins
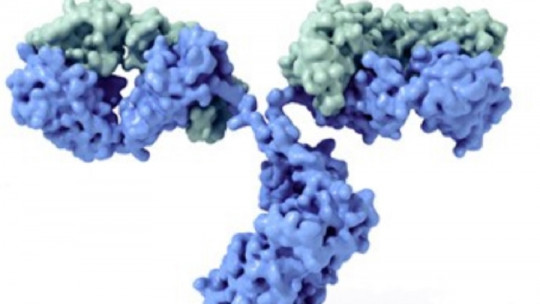
Immunoglobulins have a typical “Y” shape, composed of two different halves You must picture this conformation clearly in your mind before continuing, since we are going to rely on this pattern to describe the general conformation of the antibodies.
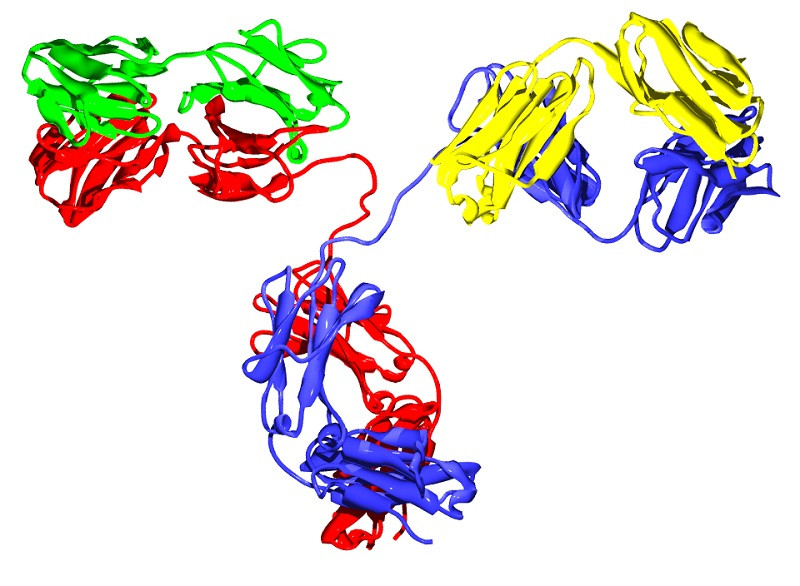
Like all proteins, An immunoglobulin has the amino acid as its basal unit, each of the subunits that, joined by peptide bonds, gives rise to peptides (less than 10 amino acids), polypeptides (more than 10) and proteins (many concatenated amino acids). In this case, the type immunoglobulin is composed of 4 polypeptide units: two heavy chains identical to each other (Heavy, at the base and cleavage of the “Y”) and 2 light chains identical to each other (Light, each of the side tips of the ramifications of the “Y”).
Each “H” region is composed of a variable region (VH) and 3-4 constant regions (CH1, CH2,CH3, etc.). On the other hand, “L” light chains are composed of a variable region (VL) and a constant region (CL). All this may sound very confusing, but it is only necessary to keep the following concept: the ends of the heavy (H) and light (L) chains are variable, while the general conformation of “Y” is constant between immunoglobulins of the same guy.
The “Y” shape is the typical one shown in biology and immunology classes, but not the only one. This monomeric form includes immunoglobulins D, E and G, while Ig A is a dimer and Ig M is a pentamer. As you can imagine, These anatomical changes also imply a clear variability in functionality
The types of immunoglobulins
We leave the molecular forest to return to slightly more general topics, this time, the different functions of immunoglobulins according to their designation. We describe them briefly.
1. Immunoglobulin A
It is found in the mucosal linings of the respiratory tract, the urogenital tract, and the lumen of the digestive system, as well as saliva, tears, and breast milk. Curiously, in the blood it is found in a monomeric form (like the “Y” described), but in the mucous membranes its arrangement is dimeric.
Due to their proximity to the only open systems within the human body (excretory, respiratory and digestive), these immunoglobulins They are the first to come into contact with viruses that invade the oropharyngeal cavity and other intestinal microorganisms
2. Immunoglobulin G
This immunoglobulin is the one most represented in the blood, cerebrospinal fluid and peritoneal fluid (of the abdominal cavity). It constitutes 80% of the total immunoglobulins so without a doubt it is the predominant one.
Furthermore, it should be noted that there are 4 subvariants of this type of immunoglobulin, from IgG 1 to IgG4. Each of them are especially skilled on a specific front, detecting antigens and toxins from different microorganisms.
3. Immunoglobulin M
It is expressed on the surface of B lymphocytes, the main effectors of the humoral response of the adaptive immune system.
They are the contingency response to an infection, since they eliminate pathogens in early stages until the immune system synthesizes enough IgG-type s. They account for 6% of circulating immunoglobulins in the human bloodstream and are present in the vast majority of animals, which is why they are considered the oldest antibodies in the evolutionary history of vertebrates.
4. Immunoglobulin E
The antibody that is clinically related to allergic symptoms. Normally, this immunoglobulin It is found in small amounts circulating in the blood, but increases drastically when the body is exposed to an allergen, or what is the same, a harmless substance that causes an unjustified reaction in the individual’s immune system. It is also expressed in atypical quantities in parasitic infections.
5. Immunoglobulin D
This is one of the immunoglobulins that is least expressed, but that does not make it any less important. It only represents 1% of the body’s total immunoglobulins and It is the largest component of the surface of many type B lymphocytes in their maturation stage Due to its rarity, its function is less defined than that of the rest of the variants already described.
Summary
As you may have seen, immunoglobulins come in various forms (isotypes) and morphological arrangements, but all of them have a very clear function: protect the body from possible infections and pathogens. From viruses to more complex morphological parasites (such as helminths), immunoglobulins are capable of recognizing them, activating the rest of the immune cells, marking them based on their surface antigens and, after the relevant cascade reaction, eliminating them.
In summary, immunoglobulins are proteins secreted by B lymphocytes and plasma cells, in response to an antigen that has infiltrated the host’s body. From immune responses to allergic reactions, antibodies have various protective functions.