The world of human biochemistry, in all its meanings, explains the metabolic processes that occur within us. Beyond ethereal concepts and self-identities, we must recognize that the human being is nothing more than a set of chemical pathways, electrical impulses, cellular respirations and polypeptide chains, at least on a purely physiological level.
Thus, thousands of small molecules explain our behaviors, habits and ailments Gastric enzymes are responsible for digestion in the stomach, the hemoglobin in red blood cells allows the transmission of oxygen to our tissues, and the release of synaptic complexes between neurons enables us to think, nothing more, nothing less. Simply put: we are the biomolecules that we synthesize, no matter how small or insignificant they may seem.
Among the entire biological conglomerate that is the human body, there are a series of proteins or conjugates responsible for very specific functions, which usually escape general attention due to the specific language they entail. Here we will see what it is myoglobin, a muscle heteroprotein that has many similarities to classical hemoglobin
What is myoglobin?
Myoglobin is a heteroprotein, that is, it is composed of a protein part (apoprotein, amino acids linked by peptide bonds) and another smaller non-protein part , the prosthetic group. The key differential characteristic between a common protein (holoprotein) and a heteroprotein is that the latter presents lipids, carbohydrates, nucleic acids and even metals in its three-dimensional structure.
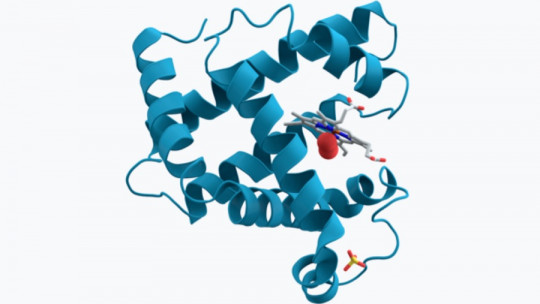
Refining the thread further, we can highlight that myoglobin is a chromoprotein type heteroprotein, since it has a metal in its chemical composition , which gives it a characteristic stain. Myoglobin is composed of a polypeptide section of 153 amino acids and a heme group containing an iron atom, just like hemoglobin. Due to this “heme” group, we can affirm that the main function of myoglobin is to store oxygen.
Furthermore, it should be noted that myoglobin It is composed of a single polypeptide chain made up of 8 alpha helices (secondary structure of the amino acid conformation), which is associated with an oxygen insertion point In the center, it has a porphyrin ring that contains iron. A proximal histidine group (His-93) associates directly with the iron molecule, while a distal histidine group (His-64) is placed on the opposite side of the formation.
In contrast, hemoglobin (responsible for transporting oxygen in the blood, within red blood cells) is composed of four different polypeptides and four oxygen binding sites, which allows for various binding kinetic properties. You could say that, From a chemical point of view, it is more “complex” than myoglobin
If we want you to have an idea of this whole conglomerate, it is the following: myoglobin is made up only of a chain of amino acids (polypeptide), which in turn is presented in the form of 8 connected alpha helices, arranged in a coiled manner on three-dimensional conformation. At the center of the heteroprotein is a heme group, with an iron molecule. If we could unwind its tertiary structure, we would see a string of 8 subunits attached to a heme ring.
Myoglobin function
Like hemoglobin, myoglobin It is a cytoplasmic heteroprotein that enables the binding of oxygen to a heme group In any case, having four polypeptide chains (globin), hemoglobin has four heme groups, which allows it to attach more oxygen to its tertiary structure. Thus, hemoglobin has more “oxygen charge”, while myoglobin has a greater affinity for it, but in a smaller quantity (only one heme group/one O2 molecule). These differences are in conjunction with the functionality of each molecule: hemoglobin transports, while myoglobin stores.
At this point, it should be noted that myoglobin concentrations are highest in the striated muscles of vertebrates , specifically, in the cytoplasm of cardiomyocytes and in the sarcoplasm of muscle fibers. Based on this premise, it can be assumed that the main function of myoglobin is to provide oxygen to the muscle mitochondria when the body is in a moment of effort, in order to avoid hypoxia at the tissue level.
In other words, myoglobin serves as a buffer for intracellular oxygen concentration and also as an O2 reserve at the muscular level. This concept is confirmed by a reality that is as curious as it is expected: animals that live in water and spend long periods of time submerged present 30 times more myoglobin in their cellular environment, compared to those that have oxygen available at all times.
These are some more functions of myoglobin, contextualized in the body’s environment :
As you can see, The functionality of myoglobin not only lies in the storage of an O2 molecule thanks to its heme group Although this is its main task, it also presents others, equally important for well-being in the cellular environment.
The role of myoglobin in clinical conditions
Myoglobin is encoded by the MB gene in humans, and like any ordered DNA sequence, it is susceptible to mutations. MB gene dysfunction has been associated with various conditions, such as compartment syndrome or medulloblastoma.
Furthermore, experimental models (knockout mice) with mutated myoglobin develop lethal cardiac diseases during fetal development. The few models that survive these conditions express compensatory mechanisms, but survival is low. Therefore, it is stated that myoglobin It is essential for the functioning of the body
Beyond conjectural grounds, myoglobin has been directly associated with a widely known clinical entity: rhabdomyolysis. In this serious condition, damage occurs to the membrane of the myocyte (muscle cell), which results in the accumulation of calcium abnormally within the muscle. This involves muscle lysis and necrosis, which in turn increases the concentration in the blood of molecules that should not be there.
Interestingly, myoglobin It is the protein that causes the most damage to the kidneys when it infiltrates the circulatory system and ends up being “filtered” into the kidney environment It is believed that this heteroprotein can precipitate in the tubules of the kidney, accumulating and causing blockages. This mechanism of toxicity explains, in part, why kidney failure is one of the main side effects of rhabdomyolysis.
Finally, it should be noted that the presence of myoglobin in the urine is measured to detect this condition, since a concentration of 100 mg/dl is capable of changing the color of the pee.
Summary
When we pay attention to a protein, enzyme or molecule related to metabolism, staying only with its structure and main function is a serious mistake. Yes, myoglobin stores oxygen to prevent muscle hypoxia, but it also has enzymatic activity, neutralizes reactive oxygen species, facilitates the diffusion of O2 into the cellular environment, and serves as one of the diagnostic criteria for rhabdomyolysis (whether in its concentration plasma or urine).
After all, every biomolecule has more than one function in our body, and if we believe that it only performs one task, it will surely be because we have not discovered the rest.