The muscular system comprises a set of more than 650 muscles that shape and support the human body. Many of these can be controlled at will, allowing us to exert enough force on the skeleton to move. For some authors, the muscular apparatus is composed only of those tissues that can move at will, while for others, involuntary muscles (heart and viscera, for example), are also included within this conglomerate.
Be that as it may, muscles allow us from movement to life itself because, without going any further, the muscular tissue of the heart (myocardium) pumps 70 milliliters of blood in each heartbeat, that is, all of the body’s blood in a short time. more than a minute. Throughout our entire lives, this titanic tissue can contract about 2,000 million times.
Whether pumping blood or performing a conscious movement, each and every muscle in our body has a specific, essential and irreplaceable function. Today we come to talk to you about the sarcomere the anatomical and functional unit of striated musculature.
types of muscles
The basic properties of all muscle tissue are contractility, excitability, extensibility and elasticity This allows the muscles to receive and respond to stimuli, stretch, contract and return to their original state so that no damage occurs. Based on these qualities, the muscular system enables the production of body movements (along with the joints), the contraction of blood vessels, the heart and production of peristaltic movements, the maintenance of posture and mechanical protection, among many others. things.
In addition to these common characteristics, it is necessary to note that There are 3 essential types of muscles We define them briefly:
Making this initial distinction is essential, since the functional unit that concerns us here (the sarcomere) is only present in the striated muscles. Now, let’s look at its properties.
What is a sarcomere?
The sarcomere is defined as the functional and anatomical unit of the striated muscle, that is, the voluntary They are a series of repeated units that give rise to morphological structures called myofibrils, and are perhaps the most ordered macromolecular structures in the entire eukaryotic cell typology. We are going to introduce many terms quickly, so do not despair, as we will go in parts.
The cells that make up striated muscle are called myofibers, and are long cylindrical structures surrounded by a plasma membrane known as the sarcolemma They are very long cell bodies, they can range from several millimeters to more than one meter (10 and 100 µm in diameter) and they have peripheral nuclei in the cytoplasm, which gives the cell a large amount of space for the contractile machinery.
If we advance in specificity, we will see that muscle myofibers contain in their sarcoplasm (cellular cytoplasm) several hundred or thousands of myofibrils, a lower level of morphological organization. In turn, each myofibril contains myofilaments, in the proportion of about 1,500 myosin filaments and 3,000 actin filaments. To give you a simple idea, we are talking about an electricity “cable” (myofiber) that, if cut transversely, contains thousands of much smaller cables inside (myofibril).
It is on this scale where we find the sarcomeres because, as we have said previously, they are the functional repeating unit that makes up the myofibrils.
Characteristics of the sarcomere
In the composition of the sarcomere Two biological elements of essential importance that we have already mentioned stand out: actin and myosin Actin is one of the most essential globular proteins in living beings, as it is one of the 3 main components of the cytoskeletons (cellular skeleton) of the cells of eukaryotic organisms.
On the other hand, myosin is another protein that, together with actin, allows muscle contraction, as it represents up to 70% of the total proteins present in this tissue. It also intervenes in cell division and vesicle transport, although such functionalities will be explored on another occasion.
The sarcomere has a very complex structure, since It is composed of a series of “bands” that move in the contractile movement These are the following:
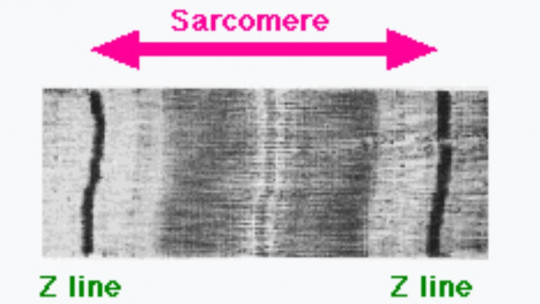
Thus, the sarcomere can be called the region of a myofibril located between two consecutive Z discs, which is approximately two microns in length. Between the Z discs there is a dark section (corresponding to the A band) where, when contracting, the thick myosin filaments and the thin actin filaments slide over each other, varying the size of the sarcomere.
Protein question
Apart from the typical contractile proteins, actin and myosin, the sarcomere contains two more large groups. We tell you about them briefly.
One of the accessory protein groups present in the sarcomere are regulatory proteins, responsible for the initiation and stopping of contractile movement. Perhaps the best known of all is tropomyosin, with a coiled structure made up of two long polypeptides. This protein regulates, together with tropin, the interactions of actin and myosin during muscle contraction.
We also observe in another block the structural proteins, which allow this very complex cellular network to remain in order and not collapse. The most important of all of them is titin, the largest protein known, with a molecular mass of 3 to 4 million Daltons (Da). This essential molecule acts by connecting the Z disk line to the M zone line in the sarcomere, contributing to force transmission in the Z line and releasing tension in the I band region. It also limits the range of sarcomere movement. when it is stressed.
Another essential structural protein is dystrophin or nebulin. The latter binds to muscle actin, regulating the extension of thin filaments. In summary, they are proteins that allow the communication of bands and discs in the sarcomere, promoting the efficient production of the complex and effective contractile movement that characterizes muscles.
Related pathologies
It is interesting to know that, when the transcription of any of these proteins fails, very severe health disorders can occur. For example, Some titin gene mutations have been associated with familial hypertrophic cardiomyopathy a congenital heart disease that affects 0.2% to 0.5% of the general population.
Another of the most popular diseases when it comes to muscles is Duchenne muscular dystrophy, caused by a defective gene for dystrophin. This is associated with intellectual disability, fatigue, motor problems and general incoordination that usually ends with the death of the patient due to associated respiratory failure. Although it may seem surprising, something as simple as a defect in protein synthesis can translate into fatal pathologies.
Summary
If you have learned anything today, it is surely that the sarcomere is an extremely complex and organized functional unit, whose structure tries to find the balance between strong and efficient contraction and biological viability (that is, everything remains in its place once produced. the movement).
Between bands, discs and lines, one thing is clear to us: sarcomeres could cover a book with just their anatomical organization. The key to movement in living beings is found in the organization of actin, myosin and other associated proteins.
- Araña-Suárez, M., & Patten, SB (2011). Musculoskeletal disorders, psychopathology and pain. Musculoskeletal Disorders Psychopathology, 1.
- Banda, A., Zona, H., Banda, I., & Discs, Z. Sarcomere: Structure and Parts, Functions and Histology.
- Bonjorn, M., Rosines, MD, Sanjuan, A., & Forcada, P. (2009). Soft tissue injuries due to friction. Biomechanics, 17(2), 21-26.
- Duchenne muscular dystrophy, Medlineplus.gov. Collected on January 10 at https://medlineplus.gov/spanish/ency/article/000705.htm#:~:text=La%20dystrophy%20muscular%20de%20Duchenne,una%20prote%C3%ADna%20en%20los %20m%C3%BAscules).
- Gómez Díaz, I. (2013). Titin in the genetic diagnosis of familial heart disease.
- Marrero, RCM, Rull, IM, & Cunillera, MP (2005). Clinical biomechanics of tissues and joints of the musculoskeletal system. Masson.
- Martín-Dantas, EH, da Silva-Borges, EG, Gastélum-Cuadras, G., Lourenço-Fernandes, M., & Ramos-Coelho, R. (2019). Concentrations and relative mobility of titin isoforms after three different flexibility trainings. Technoscience Chihuahua, 13(1), 15-23.
- Mora, IS (2000). Muscular system.
- Rosas Cabrera, RA (2006). Study of the mechanical properties of the protein titin.