How many chemical elements must exist in the universe? This question still has a long way to go to be answered, since scientists create their own artificial chemical elements from time to time.
However, what we can respond to is how many types of chemical elements must exist something we are going to find out next.
Types of chemical elements
In nature we can find all kinds of substances. These substances are not usually found in a pure state, but are the result of the combination of different elements or materials that, through different reactions, processes and periods of time, have given rise to all kinds of matter. There is nothing in the universe that is not the result of the combination of atoms belonging to different types of chemical elements which we are going to see in depth.
However, before looking at the types of chemical elements, let’s do a little review of high school natural sciences and remember what chemical elements are.
We call a “chemical element” a matter that is composed of the same type of atom, that is, a substance that is atomically pure. Chemical elements cannot be broken down into simpler ones and are classified in the periodic table of the elements as pure materials of the universe.
But be careful! We should not confuse elements with simple substances, since in certain cases two or more atoms of the same element can make up molecules grouped in different ways that cause some of the physical properties of the element in question to vary, these cases being isotopes. . For example, diamond and carbon are substances made from the chemical element carbon (C) but which is organized in different ways and results in two completely different materials.
Carl Sagan said that we are stardust reflecting on stars. This beautiful phrase is not a mere metaphor, but a scientific fact. Chemical elements are formed, as far as we know, inside stars, being the result of complex processes of fusion and atomic fission that generate increasingly heavier elements, the result of a process called nucleosynthesis.
Most of the known elements can be obtained from nature , finding themselves spontaneously or forming compounds with other elements such as uranium (U), carbon (C), silicon (Si), silver (Ag) or gold (Au). Others, however, have been manufactured in laboratories, such as americium (Am), berkelium (Bk) or curium (Cm). Whatever its method of obtaining or whether it is present in nature, depending on its properties, the chemical element in question will have one use or another.
Currently, there are about 118 chemical elements known, although taking into account that humans have been able to manufacture new elements, it is a matter of time before the periodic table expands.
The main types of chemical elements
The main types of chemical elements are represented in the periodic table, a classification system created by the Russian chemist Dmitri Mendeleev (1834-1907) who laid its foundations in 1869. The chemical elements are arranged visually based on their properties and characteristics.
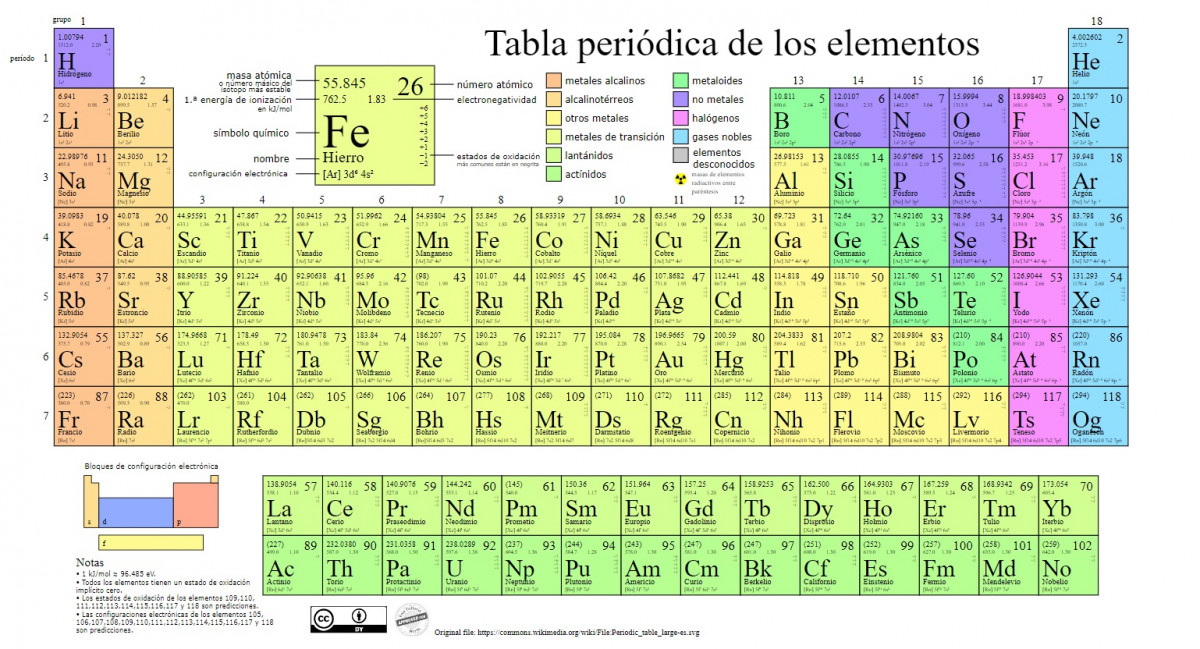
As time went by and as chemistry made important scientific discoveries, this table would be expanded consecutively reaching the form it has today with the 118 elements known so far.
Currently, in this table we can find the following types of chemical elements:
1. Metals
The metals are chemical elements that generally contain between one and three electrons in the last orbit of their atom, electrons which they can easily give up converting them into conductors of heat and electricity.
Metals are usually malleable and ductile, with a characteristic shine whose intensity depends on the movement of the electrons that make up their atoms. In most cases, metals are solid at room temperature, except for mercury.
Among the metals we find gold (Au), silver (Ag), copper (Cu) and aluminum (Al) whose physical characteristics make them magnificent conductors of electricity although their presence in nature is very varied, reflected in the difference between their weights.
It is believed that 75% of the chemical elements existing in nature are metals, while the remaining 25% would be made up of noble gases, metalloids and other types.
There are classifications within this category, including actinides, lanthanides, transition metals, alkali metals, alkaline earth metals and other metals.
1.1. Lanthanides
Lanthanide elements are found in deposits made up of many minerals. They are white metals that oxidize easily when they come into contact with air Among them we find lanthanum (La), promethium (Pm), europium (Eu) and ytterbium (Yb).

1.2. Actinides
All actinide isotopes are radioactive. Among them we find actinium (Ac), uranium (U), plutonium (Pu) and einsteinium (Es).
1.3. Transition metals
Transition metals are located in the central part of the periodic system. Its main characteristic is that have electronic configuration of the “d” orbital partially filled with electrons
In this group there are substances of all types and, according to its broadest classification, it would correspond to the chemical elements from 21 to 30, from 39 to 48, from 71 to 80 and from 103 to 112, being a total of forty and among them we would find vanadium (V), ruthenium (Ru), silver (Ag), tantalum (Ta) and lawrencium (Lr).

1.4. Alkali metals
The alkali metals are a group of six elements composed of lithium (Li), sodium (Na), potassium (K), rubidium (Rb), cesium (Cs) and francium (Fr) They are shiny, soft metals, highly reactive at normal temperature and pressure and easily lose their external electron, located in their “s” orbital.
1.5. Alkaline earth
Alkaline earth metals are a group of elements in which we find beryllium (Be), magnesium (Mg), calcium (Ca), strontium (Sr), barium (Ba) and radium (Ra). Its name comes from the name given to its oxides, “earths”, which have basic or alkaline properties.
The alkaline earth They are harder than alkaline ones, they shine and are good electrical conductors They are less reactive than alkali and act as good reducing agents. They have the ability to form ionic compounds and all of them have two electrons in their outermost shell.

1.6. Other metals
The “other metals” category is a type of substances that are found here because we don’t really know where to put them
They are metallic elements located in the periodic table along with metalloids, within the p block. They tend to be soft with low melting points. Among them are aluminum (Al), indium (In), tin (Sn) and bismuth (Bi) among others.

2. Non-metals
Non-metals generally have between five and seven electrons in their last orbit, a property which causes them to gain electrons instead of giving them up and, thus, they manage to have eight electrons which stabilizes them as atoms.
This elements They are very poor conductors of heat and electricity Added to this, they do not have a characteristic shine, they are not very malleable, not very ductile and are very fragile in the solid state. They cannot be laminated or stretched, unlike metals.
Most of them are essential for biological systems, since they are present in organic compounds, such as sulfur (S), carbon (C), oxygen (O), hydrogen (H) and iodine (I). ).

3. Metalloids
As their name suggests, metalloids are an intermediate classification between metals and non-metals that have properties of both groups This is because they have four atoms in their last orbit, an intermediate amount to that possessed by metals and non-metals.
These chemical elements conduct electricity only in one direction, not allowing it to be done in the opposite direction as occurs in metals. We have an example of this in silicon (Si), a metalloid used in the manufacture of semiconductor elements for the electronic industry thanks to this property.
Other metalloids are: boron (B), arsenic (As), antimony (Sb) and polonium (Po).

4. Halogens
Halogens are a group of six elements that They tend to form molecules composed of two atoms (diatomic) that are very chemically active due to their electronegativity
These substances usually occur in the form of ions, that is, electrically charged molecules, which in this case are mononegative, highly oxidizing. This means that halogens are caustic and corrosive substances.
The halogens are: fluorine (F), chlorine (Cl), bromine (Br), iodine (I), astatine (At) and tenese (Ts).

5. Noble gases
The noble gases are a group of seven whose natural state is gaseous. They usually occur in the form of diatomic molecules with very low reactivity, that is, they do not react with other elements, composing other substances and, for this same reason, they are known as inert gases. This is because in its last orbit there are the maximum number of electrons possible for that level, eight in total.
This select group of elements is made up of helium (He), neon (Ne), argon (Ar), krypton (Kr), xenon (Xe), radon (Rn) and oganeson (Og), formerly known as ununoctium.